Sepiidae
Katharina M. Mangold (1922-2003) and Richard E. Young

This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
The long, oval body is flattened dorsoventrally and bordered all along by narrow fins that do not connect at the posterior end. The internal shell (i.e. sepion or cuttlebone) lies dorsally in the body beneath the skin. The shell is a thick, oval, lanceolate or rhomboidal calcareous structure containing numerous gas and/or water filled chambers. The shell enables buoyancy control (Denton and Gilpin-Brown, 1973; Denton, 1974; see Mangold and Bidder, 1989). The eye lenses are covered by a protective cornea. The ventral arms are generally the longest and broadest; the left ventral is hectocotylized in males. The arms bear suckers in 2 to 4 series. The tentacles are completely retractile into pockets.


Figure. Side view of Sepia apama. Photograph by Mark Norman.
Brief diagnosis:
A sepioid ...- with a cuttlebone.
- with body somewhat flattened dorsoventrally.
Characteristics
- Arms
- Arms IV flattened ventrally with broad lateral margins extending onto head.
- Left arm IV hectocotylized.
- Tentacles
- Tentacles, including clubs, completely retractile into pockets occupying ventral region of head. (More information on the tentacular club is found here.)
- Tentacles, including clubs, completely retractile into pockets occupying ventral region of head. (More information on the tentacular club is found here.)
- Head
- Eye pore inside of ventral eyelid.
- Eye pore inside of ventral eyelid.
- Funnel
- Funnel locking-apparatus short, oval to ear-shaped.
- Funnel locking-apparatus short, oval to ear-shaped.
- Mantle
- Dorsal mantle margin free from head.
- Mantle adductor absent.
- Fins
- Fins narrow (length ca. 4 or more times individual fin width), extend almost full length of mantle.
- Fins attach to shell posteriorly and to mantle anteriorly.
- Attachment of posterior fin-lobes adjacent but separate.
- Shell
- Shell a cuttlebone.
Nomenclature
A list of all nominal genera and species in the Sepiidae can be found here. The list includes the current status and type species of all genera, and the current status, type repository and type locality of all species and all pertinent references.
Life History
The sepiids are benthic or benthopelagic. The young resemble the adults when they hatch and are also benthic. The sepiids have large eggs which are stored in the oviduct. During spawning the eggs are coated with oviducal gland secretion, then with nidamental gland secretion and the female deposits them singly on a substrate (see Boletzky, 1983). The duration of embryonic development depends upon temperature.
The young hatch with a yolk reserve that allows them to survive for a few days in the absence of food. When food, especially mysids, is available, the hatchlings begin to attack immediately. Swimming prey are seized by the tentacular clubs. Benthic prey, such as crabs, are pounced upon and seized with the arms. While crustacea are the main prey in younger Sepia, subadults and adults also eat fishes (Guerra, 1985; Castro and Guerra, 1989, 11990).
Growth is fast. The conversion rate is 30 to 40% (Pascual, 1978). In warm waters, animals grow faster and mature at a smaller size than those living in cold (temperate) waters. The shell or cuttlebone grows by adding new chambers bounded by lamellae (the septa of the striated zone) which are visble on the posterior ventral surface of the cuttlebone. In tropical species, a chamber may be formed daily so that the number of septa corresponds to the age in days. In temperate water however, it takes two to three days for a new chamber to be completed. Sepia officinals hatches with a cuttlebone of about 10 septa (Boletzky, 1983).
Spawning takes place over a prolonged period of time or throughout the year. Spawning is intermittent (Boletzky, 1975; Mangold et al., 1993). The number of mature eggs found in the ovary at any one time is not an indicator of fecundity. In Sepia officinalis the total of laid eggs surpasses by several times the holding capacity of the ovary. The endocrine and nervous control mechanisms of reproduction, are complicated (Boucaud-Camou et al., 1994).
Spawning migrations exist in temperate water species but are often absent in tropical ones. The European cuttlefish, Sepia officinalis migrates south-north (Atlantic Ocean, North Sea) or offshore-inshore (Mediterranean) for spawning. The life-span of sepiids varies between a few months and 1 to 2 or 3 years according to the adult size and the environment (temperature, food availability).
Habitat
The genus Sepia includes numerous species (more than 100) that live in tropical, subtropical and temperate waters in all oceans and seas except the coasts of the Americas (for geographic distribution of the different species see Adam, 1979; Adam and Rees, 1966; Boletzky et al., 1996; Nesis, 1982; Roeleveld, 1972).
The two other genera of the family, Metasepius and Sepiella, occur only in South African waters and in the Western Pacific.
The members of this family are benthic and inhabit primarily coastal waters but are also found on the slope to a depth of about 500 meters.
Behavior
Hatching occurs at nighttime (Paulij et al., 1991). The hatchlings hide during the day and actively seek prey in the water column during the night, as do the adults. Laboratory reared hatchlings of Sepia officinalis released into the sea are difficult to locate as their camouflage is very effective (Hanlon and Messenger, 1988; Roper & Hochberg, 1988). The chromatophoric system, as well as the chromatophore lobes of the brain, are well developed in hatchlings. In hatchlings there are 400 to 500 chromatophores per mm2 of skin; in large adults, ahowever, there are only 35 to 50 per mm2 of skin (Hanlon & Messenger, 1988). During daytime hatchlings bury in the sand or they fix themselves on a substrate by means of a "sucker" formed by the antero-ventral surface of the mantle and the postero-ventral surface of the large ventral arms (Boletzky, 1974; Hanlon and Messenger, 1988) as do the adults.
Wells (1958, 1962) has shown in learning experiments that hatchlings attack mysids presented behind a glass, although there is no reward. Prey recognition is innate which gives the hatchlings a built-in-mechanism that assures attacks on mysids. Also, like the adults, hatchlings do not follow a prawn that passes out of sight (Messenger, 1973). There is no evidence of learning in hatchlings and very young animals. At two months of age there is improvement but retention is still poor. At four months of age, learning, retention and hunting behaviour are exhibited (Messenger, 1973, 1977). The vertical lobe system which is concerned with learning and memory (see Young, 1965) is less developed in hatchlings that it is in adults (Froesch, 1971; Wirz, 1959).
Fisheries
The sepiids are commercially fished in many areas of the world (e. g. the Mediterranean and the west coast of Africa).
Distribution
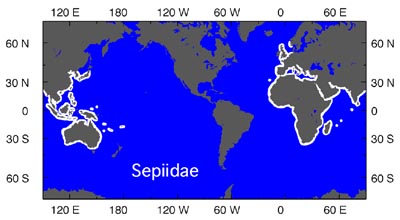
The Sepiidae have a tropical/temperate distribution. They are mostly shallow-water animals although they are known from depths of about 600 m (Lu and Roper, 1991). They have an unusual biogeographic pattern (seen below, in white) in which they are absent from the Americas. Young, et al. (1998) suggest that by the time the family evolved in the Old World, the northern migration bridge across the Atlantic was closed to these warm water species.
References
Adam, W. 1979. The Sepiidae (Cephalopoda, Decapoda) in the collections of the Western Australian museum. Rec. West. Aust. Mus. 7(2):111-212.
Adam, W. and W. J. Rees. 1966. A review of the cephalopod family Sepiidae. Sci. Rep. John Murray Exped. 11: 1-165.
Boletzky, S.V. 1983. Sepia officinalis, pp. 31-52. In: Cephalopod Life Cycles Volume I, Boyle, P.R. (ed.), Academic Press, London, 475 pp.
Boucher-Rodoni, R., and Mangold, K. 1985. Ammonia exretion during feeding and starvation in Octopus vulgaris. Mar. Biol. 86:193-197.
Castro, B.G., and Guerra, A. 1989. Feeding pattern of Sepia officinalis in the Rio de Vigo. Jour. Mar. Biol. Ass. U. K. 69:545-553.
Castro, B.G., and Guerra, A. 1990. The diet of Sepia officinalis (Linnaeus, 1758) and Sepia elegans (d'Orbigny, 1835) (Cephalopoda, Sepioidea) from the Ria de Vigo (NW Spain). Sci. Mar. 54(4):375-388.
Froesch, D. 1971. Quantitative untersuchungen am zentralnervensystem der Schlupfstadien von zehn Mediterranen cephalopodenarten. Rev. Suisse Zool. 78(4)(57):1069-1122.
Guerra, A. 1985. The cephalopod fisheries in two upwelling areas off the West Coast of Africa: a comparison. Simp. Int. O Afl., Inst. Inv. Pesq., Barcelona 11:749-760.
Hanlon, R. T. and J. B. Messenger.(1988. Adaptive coloration in young cuttlefish (Sepia officinalis L.): the morphology and development of body patterns and their relation to behaviour. Phil. Trans. Roy. Soc. Lond. B 320: 437-487.
Roper, C.F.E., and F.G. Hochberg. 1988. Behavior and systematics of cephalopods from Lizard Island, Australia, based on color and body patterns. Malacol. 29(1):153-193.
Khromov, D. N., C. C. Lu, A. Guerra, Zh. Dong and S. v. Boletzky. 1998. A synopsis of Sepiidae outside Australian waters. Smithson. Contr. Zool., 586: 77-156.
Lu, C. C. . A synopsis of Sepiidae in Australian waters. Smithson. Contr. Zool., In press.
Lu, C. C. and C. F. E. Roper. 1991. Aspects of the biology of Sepia cultrata from southeastern Australia. In: La Seiche, The Cuttlefish. Boucaud-Camou, E. (Ed). Caen, France; Centre de Publications de l'Université de Caen: 192-192.
Mangold, K. 1989. Cephalopodes. Traité de Zoologie. Tome V. Masson, Paris. 804pp.
Mangold, K. M., R. E. Young and M. Nixon. 1993. Growth versus maturation in cephalopods. p. 697-704. In: T. Okutani, R. K. O'Dor and T. Kubodera (eds.). The Recent Advances in Cephalopod Fishery Biology. Tokai University Press. Tokyo.
Messenger, J.B. 1973. Learning in the cuttlefish, Sepia. Anim. Behav. 21:801-826.
Wells, M.J. 1962. Early learning in Sepia. Symp. Zool. Soc. Lond. 8:149-169.
Wells, M.J. 1958. Factors affect reactions to Mysis by newly hatched Sepia. Behav. 13:96-111.
Wirz, K. 1959. Biometric studies of the cephalopod nervous system. Bull Biol. France Belgique 43:71-117.
Young, R. E., M. Vecchione and D. Donovan. 1998. The evolution of coleoid cephalopods and their present biodiversity and ecology. South African Jour. Mar. Sci.., 20: 393-420.
Title Illustrations

| Scientific Name | Sepia officinalis |
|---|---|
| Location | Banyuls-sur-Mer, France |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0.
|
| Copyright |
© 1996

|
About This Page
Katharina M. Mangold (1922-2003)

Laboratoire Arago, Banyuls-Sur-Mer, France

University of Hawaii, Honolulu, HI, USA
Page copyright © 2019 Katharina M. Mangold (1922-2003) and
 Page: Tree of Life
Sepiidae .
Authored by
Katharina M. Mangold (1922-2003) and Richard E. Young.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Sepiidae .
Authored by
Katharina M. Mangold (1922-2003) and Richard E. Young.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- Content changed 29 August 2016
Citing this page:
Mangold (1922-2003), Katharina M. and Richard E. Young. 2016. Sepiidae . Version 29 August 2016 (under construction). http://tolweb.org/Sepiidae/19987/2016.08.29 in The Tree of Life Web Project, http://tolweb.org/




 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site