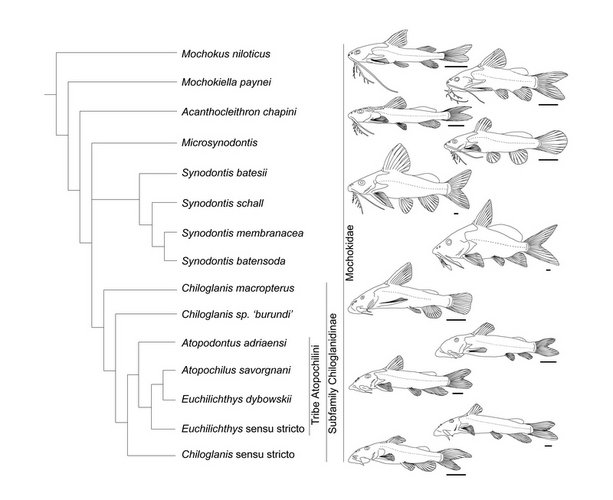
Mochokidae
African Squeaker and Suckermouth Catfishes
John P. Friel and Thomas R. Vigliotta

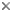
This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
The Mochokidae are a family of African catfishes known commonly as ‘squeakers’ and ‘upside-down catfishes.’ These common names refer to some unusual habits of certain members of the large genus Synodontis. The name squeaker refers to the fact that, when agitated, many species in the genus are capable of making a squeaking noise by stridulation of the pectoral spine against the pectoral girdle (Jubb, 1967); stridulation is also apparent in Mochokiella paynei and some species of Atopochilus.
Even more peculiar is the habit of some species of Synodontis that are known to swim upside-down. This habit seems to be correlated with feeding while upside-down at the water’s surface (Bishai & Abu Gideiri, 1965b), but upside-down catfishes will rest and swim in the inverted position on a regular basis. Chapman et al. (1994) showed that an upside-down posture near the surface also facilitates respiration in poorly oxygenated water. While the genus Synodontis presents the most well-known species with their fascinating behaviors and natural histories, the family is actually much more interesting when taken as a whole.
Mochokid catfishes are currently restricted to the freshwaters of Africa, but are nearly ubiquitous in the habitable waters of the continent. A high degree of morphological diversity allows mochokid catfishes to inhabit some of the fastest flowing streams and cataracts to the widest and deepest stretches of the Congo River. Mochokids also inhabit the massive African rift lakes like Tanganyika, Victoria and Nyasa. The greatest diversity of mochokids almost certainly occurs in the Congo River and its numerous tributaries, but they are also found in many of the rivers and lakes of western Africa, southern Africa, eastern Africa and in the Nile. Like a handful of other catfishes, some mochokids are known to swim in mid-water; other members of the family are primarily benthic. Likewise, some mochokids shoal while others are rather solitary. As a rule they are most active during the night, but they can be found hiding amongst plants, logs and other submerged structure during the day.

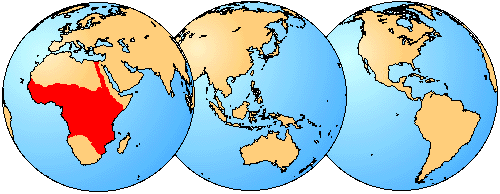
Geographic distribution of extant species of the Mochokidae.
Fossil mochokids, of the genus Synodontis, have been found in deposits from eastern and northern Africa dating to at least the early Miocene (at least 20 mya) (Stewart, 2001). Interestingly, fragments of pectoral spines of Synodontis dating from the early Oligocene have been found in Oman, an area where mochokids do not exist today (Otero & Gayet, 2001). Fossil mochokids outside of the genus Synodontis are presently unknown.
In all, nearly 250 species of mochokid catfishes have been described, but a large number of those names are now considered junior synonyms. The number of valid, described species is approaching 200 and several undescribed species are known to exist. This makes the Mochokidae one of the largest families of catfishes and certainly the largest family of African catfishes. The nearly 200 species are placed in only nine genera. The largest genus in the family is Synodontis, with approximately 120 species. The genus Chiloglanis follows with about 45 species and numbers for the remaining genera are rather paltry in comparison. Ferraris (2007) provides the most recent list of mochokid species and synonymies (188 listed as valid). Synodontis acanthoperca Friel & Vigliotta (2006), Chiloglanis productus Ng & Bailey (2006), Synodontis grandiops, S. ilebrevis and S. lucipinnis (Wright & Page, 2006), Atopodontus adriaensi (Friel & Vigliotta, 2008), Synodontis kogonensis and S. ouemeensis (Musschoot & Lalèyè, 2008), Synodontis macropunctata (Wright & Page, 2008), Synodontis ngouniensis (De Weirdt et al., 2008), Synodontis orientalis (Seegers, 2008) and Synodontis woleuensis (Friel & Sullivan, 2008) were described too recently to be included in that work. Wright & Page (2006) also make some notable taxonomic changes, including the resurrection of two species of Synodontis.
Little is known about the ecology of most mochokids. What is known pertains mostly to diet and is biased towards species of Synodontis. Stomach contents from Synodontis have included insects, nematodes, crustaceans, mollusks, annelids, seeds, algae, diatoms, fish scales and, incidentally, sand (Bishai & Abu Gideiri, 1965a; Bishai & Abu Gideiri, 1965b; Winemiller & Kelso-Winemiller, 1996; Sanyanga, 1998). From observations of stomach contents, the diet of most mochokids is probably very similar; that is, they are omnivores. For some of the larger sucker-mouthed species (i.e., Atopochilus and Euchilichthys) the stomach contents contain a high proportion of silt, algae and detritus. For these taxa it seems likely that scraping or grazing is the predominant method of feeding.
Beyond information gleaned from captive breeding of certain species of Synodontis, very little is known about reproduction in mochokids. The best studied mochokids in this regard are most likely Nile River Synodontis (including S. membranacea and S. batensoda, previously placed in other genera). Still, details are limited; the studies indicate that spawning occurs from July to October, which coincides with the flooding season, and that pairs swim in unison during spawning bouts (Bishai & Abu Gideiri, 1968). The most interesting and detailed information on mochokid reproduction relates to a species from Lake Tanganyika, Synodontis multipunctatus, which is a brood parasite of mouth brooding cichlids such as Simochromis and Haplochromis (Sato, 1986; Wisenden, 1999). Adults of this species spawn in the midst of spawning cichlids and the fertilized catfish eggs are taken into the mouth of a cichlid. The catfish eggs hatch first and will eat the host eggs before they leave the host mouth. Most amazingly, this species has been able to parasitize mouth brooding cichlids from South America in captivity (Loiselle, 1998).
Characteristics
In addition to being numerous and widespread on the African continent, the Mochokidae are actually quite diverse in terms of morphology. Mochokids are small to medium sized catfishes, ranging from less than 40 mm SL as an adult in certain species of Chiloglanis and Microsynodontis, up to a reported 800 mm SL in some species of Synodontis. Most of the larger species are in the genus Synodontis; still, the majority of those are less than 300 mm SL.
The morphology of mochokids is notably diverse aside from size as well; the size and shape of the mouth and the shape of the body are notable examples. Most mochokids exhibit a ventrally directed mouth, with papillose lips and, more often than not, branched mandibular barbels (e.g., most Synodontis ). Species exhibiting this type of mouth are quite often deep bodied and somewhat triangular in lateral view, their greatest depth being at the base of the dorsal spine. Yet others with this same type of mouth tend to be more cylindrical along their entire length (Microsynodontis and few Synodontis). Species of Chiloglanis, Atopodontus, Atopochilus and Euchilichthys exhibit a ventrally directed mouth, but it is formed as a sucker-like oral disc, wherein the lower lip is greatly expanded and incorporates the mandibular barbels. Here, the body shape tends to be quite cylindrical or depressed and the head is quite often greatly depressed. Some mochokids do not possess a ventrally directed mouth. Mochokiella paynei, Acanthocleithron chapini and members of the genus Mochokus have mouths that are only slightly subterminal; while the mandibular barbels are still branched, the lips are not nearly as fleshy. Here, again, the body shape tends to be relatively cylindrical.


Lateral view of Atopochilus vogti, illustrating typical body shape in sucker-mouthed mochokids; CU 93752. ©
Pigmentation and patterning of mochokid skin is also diverse. Mochokids are popular in the pet trade because they have showy colors, sharply contrasting patterns like stripes and polka-dots and, quite often, extravagant fins. As some of the largest and most active mochokids, species of Synodontis display an amazing array of patterns and pigmentation that may, in some cases, serve as visual cues to conspecifics. It might also be true that the bright, contrasting coloration found in some species serves as a warning to predators. Like many catfishes, some mochokids possess specialized poison glands for delivering offensive chemicals along with a ‘stick’ by the pectoral spine; anecdotal accounts indicate that these wounds can be very painful. However, field studies that might demonstrate function of these varied patterns have not been done.


Lateral view of Synodontis ornatipinnis, illustrating the striking patterns seen in some species of Synodontis; CU 91403. ©
Based largely on coloration and pattern some scientists and aquarists have recognized ‘species flocks’ of Synodontis. The largest and most visually impressive of these is the Lake Tanganyika Synodontis species flock. The flock consists of at least six species, all endemic to the lake, that show amazingly similar coloration and patterning. Recent work suggests that this ‘flock’ is not monophyletic and that the biogeographic scenario is more complicated than a simple radiation after lake formation (Day & Wilkinson, 2006; Koblmuller et al., 2006). Regardless, in this group of species, the nearly constant color pattern consists of a light brown base color and darker brown polka-dots. It is made more impressive by the fins, which have dark brown membranes basally and stark white trailing edges. The cryptic nature of Lake Tanganyika Synodontis has led to an underestimate of the actual number of species present (Wright & Page, 2006); three new species were described and two others were resurrected in that work. There are several other smaller flocks with their own unique patterns and pigmentation outside of the lake, and cryptic species probably exist for these as well.
Many mochokid species exhibit obvious sexual dimorphism. For example, many species in the genus Chiloglanis show dimorphism of the caudal and anal fins (Roberts, 1989; Seegers, 1996; Friel & Vigliotta, 2006); some Chiloglanis also display sexual dimorphism of the cleithral process, wherein males possess a greatly enlarged process shielding the flank. In the closely related genus Atopochilus, sexual dimorphism of the anal fin is sometimes evident. Some mochokids exhibit spiny ornamentation of the skull roof bones, opercular series and pectoral girdle (Friel & Vigliotta, 2006). In the case of Synodontis acanthoperca, a spine found at the rear of the opercle is, itself, sexually dimorphic. The spines of males are much larger than those of females. This is also true for Mochokiella paynei (personal observation), which was previously unknown to possess opercular spines or exhibit sexual dimorphism.

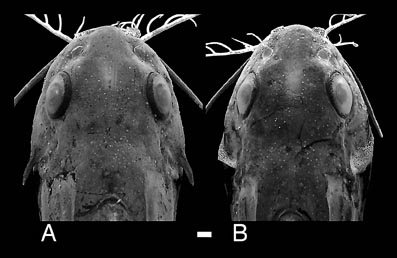
Dorsal view of heads illustrating sexual dimorphism of the opercular spine in Synodontis acanthoperca: A. CU 89005, male holotype, 44.1 mm SL; B. CU 89006, female paratype, 40.4 mm SL. The pectoral spines in both specimens have been removed from the images to make the margins of the head more distinct against the background. Scale bar equals 1 mm. ©
Diagnosis
Vigliotta (2008) provides several synapomorphies as diagnostic features for the Mochokidae including: absense of the ascending process of Meckel’s cartilage; a shortened horizontal process of Meckel’s cartilage; coronomeckalian extremely reduced or even absent; absence of a coronoid process; absence of an interhyal; presence of a pectoral locking foramen (absence/reversal in most suckermouthed species); seven pelvic-fin rays; fusion of upper caudal-fin elements (absence/reversal in Atopochilus and Euchilichthys); a reduced number of mandibular sensory canal pores (3 or fewer); and distinctive, ramified inner and outer mandibular barbels (absence/reversal in suckermouthed mochokids where these barbels are partially or completely incorporated into an expanded lower lip).
Discussion of Phylogenetic Relationships
Nine genera and approximately 200 valid species are currently recognized. Some fossilized pectoral spines have been attributed to Synodontis, but none of them are described as new. Phylogenetic relationships among the mochokid genera were investigated by Vigliotta (2008) who found that Chiloglanis is possibly a paraphyletic assemblage, that Synodontis must include S. membranaceous and S. batensoda (formerly placed in Hemisynodontis and Brachysynodontis respectively) and that the reciprocal monophyly of Atopochilus and Euchilichthys are questionable at best; all remaining genera are recovered as valid and monophyletic. The general relationships between mochokid genera are illustrated in the phylogeny below.
Key to Genera
Following key modified from Vigliotta (2008)
- 1a. Lips and barbels modified into oral disc (Fig. 27C); postcleithral process short (Fig. 22C); 5 infraorbitals (Figs. 2C, 4B); pelvic-fin origin at vertical at end of dorsal-fin base...2
- 1b. Lips and barbels not modified into oral disc; postcleithral process quite long (Fig. 22B); 4 infraorbitals Figs. 2B, 4A); pelvic-fin origin beyond end of dorsal-fin base...5
- 2a. Mandibular teeth bunched (in bouquet) at midline (Fig. 3D in Friel and Vigliotta, 2008) in or spread across mouth opening in one or two discrete rows (Fig. 3E–F in Friel and Vigliotta, 2008); eyes without free orbital margin; mandibular sensory-canal absent; 4 to 6 dorsalfin rays (typically 5); 5 to 7 branchiostegal rays (typically 5 or 6)…Chiloglanis
- 2b. Mandibular teeth spread across mouth opening in more than two discrete rows (Fig. 3A–C in Friel and Vigliotta, 2008); eyes with free orbital margin; mandibular sensory canal present, with 2 pores on each side; 6 to 7 dorsal-fin rays; 7 to 8 branchiostegal rays...3
- 3a. Small anteriorly directed pocket underneath lower lip produced by folds of skin (Fig. 4A in Friel and Vigliotta, 2008); width of mandibular tooth rows less than 66% width of paired premaxillae (Fig. 3A in Friel and Vigliotta, 2008); caudal fin emarginate; gas bladder extremely reduced to two small bulbs (Fig. 5)…Atopodontus
- 3b. Small anteriorly directed pocket underneath lower lip absent (Fig. 4B in Friel and Vigliotta, 2008); width of mandibular tooth rows more than 66% width of paired premaxillae (Fig. 3B–C in Friel and Vigliotta, 2008); caudal fin forked; gas bladder only modestly reduced (Fig. 3C)...4
- 4a. Mandibular teeth spatulate and unicuspid (Fig. 10C); large posterior pectoral-spine serrae; one or only a few pores at sites along the cephalic sensory canals; fewer than 40 vertebrae…Atopochilus
- 4b. Mandibular teeth with lengthwise keel creating trowel shape and sometimes bicuspid from wear (Fig. 10A); small posterior pectoral-spine serrae; several pores at various sites along the cephalic sensory canals; more than 40 vertebrae…Euchilichthys
- 5a. S-shaped auxiliary dentary teeth present (Fig. 10A, C–F); premaxillary teeth differentiated by shape and size front to back; lips plicate (with folds at corners of mouth)...6
- 5b. S-shaped auxiliary dentary teeth absent (Fig. 10B); premaxillary teeth showing little, if any, differentiation from front to back; lips papillose, but not plicate (without folds)...7
- 6a. Eyes with free orbital margin; 17 principal caudal-fin rays (only 13 in Synodontis contracta); tail forked; 6 to 10 pectoral-fin rays (usually 8 or 9); lateral mandibular barbels with single, gracile branches at each point along length or doubly branched (Fig. 27A–B)…Synodontis
- 6b. Eyes without free orbital margin; 12 to 14 principal caudal-fin rays; tail truncate or rounded; 6 or 7 pectoral- fin rays (typically 6); lateral mandibular barbels with single, gracile branches at each point along length (Fig. 27A)…Microsynodontis
- 7a. Dorsal surface of the head and nuchal shield covered by large ridges and spinous projections; cleithrum bearing spine in males; rounded, blunt postcleithral process; anus and urogenital opening distant; free orbital margin present; gill openings open to isthmus; tips of mandibular teeth spatulate; medial mandibular barbels with multiple, thick branches at each point along length (Fig. 27B); 8 to 9 pectoral-fin rays; 17 caudal-fin rays; more than 40 vertebrae…Acanthocliethron
- 7b. Dorsal surface of the head and nuchal shield without ridges and spinous projections; cleithrum without spine in males; pointed postcleithral process; anus and urogenital opening very close; free orbital margin absent; gill openings restricted to sides of the head; tips of mandibular teeth pointed; medial mandibular barbels with single, gracile branches at each point along length (Fig. 27A); 5 to 7 pectoral-fin rays; 13 or 15 caudal-fin rays; fewer than 36 vertebrae...8
- 8a. Adipose fin with rays; tips of pelvic fins not reaching origin of anal fin; opercle without obvious spine in males; 5 to 6 pectoral-fin rays; 2 mandibular sensory-canal pores on each side…Mochokus
- 8b. Adipose fin without rays; tips of pelvic fins just reaching origin of anal fin; opercle bearing obvious spine in males; 7 pectoral-fin rays; 3 mandibular sensory-canal pores on each side…Mochokiella
References
Bishai H.M. and Y.B. Abu Gideiri. 1965a. Studies on the biology of genus Synodontis at Khartoum. I. Age and Growth. Hydrobiologia 26:85–97.
Bishai H.M. and Y.B. Abu Gideiri. 1965b. Studies on the biology of genus Synodontis at Khartoum. II. Food and Feeding Habits. Hydrobiologia 26:98–113.
Bishai H.M. and Y.B. Abu Gideiri. 1968. Studies on the biology of genus Synodontis at Khartoum. III. Reproduction. Hydrobiologia 31:193–202.
Chapman, L.J., L. Kaufman, and C. A.Chapman. 1994. Why swim upside-down - a comparative study of 2 mochokid catfishes. Copeia 1994:130–135.
Day, J.J., and M. Wilkinson. 2006. On the origin of the Synodontis catfish species flock from Lake Tanganyika. Biology Letters 2:548–552.
De Weirdt, D., E. Vreven, and Y. Fermon. 2008. Synodontis ngouniensis, a new species (Siluriformes: Mochikidae) from Ngounié and Nyanga basins, Gabon and Republic of Congo. Ichthyological Exploration of Freshwaters 19(2):121–128.
Ferraris, C.J., Jr. 2007. Checklist of catfishes, recent and fossil (Osteichthyes: Siluriformes), and catalogue of siluriform primary types. Zootaxa 1418:1–628.
Friel, J.P., and J.P. Sullivan. 2008. Synodontis woleuensis (Siluriformes: Mochokidae), a new species of catfish from Gabon and Equatorial Guinea, Africa. Proceedings of the Academy of Natural Sciences of Philadelphia 157:3–12.
Friel, J.P. and T.R. Vigliotta. 2006. Synodontis acanthoperca, a new species from the Ogôoué River system, Gabon with comments on spiny ornamentation and sexual dimorphism in mochokid catfishes (Siluriformes: Mochokidae). Zootaxa 1125:45–56.
Friel, J.P. and T.R. Vigliotta. 2008. Atopodontus adriaensi, a new genus and species of African suckermouth catfish from the Ogôoué and Nyanga River systems of Gabon (Siluriformes Mochokidae). Proceedings of the Academy of Natural Sciences of Philadelphia 157:13–23.
Jubb, R.A. 1967. Freshwater fishes of Southern Africa. Cape Town, Amsterdam, Balkema, 248 pp.
Koblmüller, S., C. Sturmbauer, E. Verheyen, A. Meyer, and W. Salzburger. 2006. Mitochondrial phylogeny and phylogeography of East African squeaker catfishes (Siluriformes: Synodontis). BMC Evolutionary Biology 6:49.
Musschoot, T., and P. Lalèyè. 2008. Designation of a neotype for Synodontis schall (Bloch and Schneider, 1801) and description of two new species of Synodontis (Siluriformes: Mochokidae). Journal of Natural History 42(17-18):1303–1331.
Ng, H.H. and R.M. Bailey. 2006. Chiloglanis productus, a new species of suckermouth catfish (Siluriformes: Mochokidae) from Zambia. Occasional Papers of the University of Michigan Museum of Zoology 738:1–13.
Otero, O. and M. Gayet. 2001. Palaeoichthyofaunas from the Lower Oligocene and Miocene of the Arabian Plate: palaeoecological and palaeobiogeographical implications. Palaeogeography Palaeoclimatology Palaeoecology 165:141–169.
Roberts, T.R. 1989. Systematic revision and description of new species of suckermouth catfishes (Chiloglanis, Mochokidae) from Cameroun. Proceedings of the California Academy of Sciences Series 4, 46:151–178.
Sanyanga, R.A. 1998. Food composition and selectivity of Synodontis zambezensis (Pisces: Mochokidae) in Lake Kariba, and the ecological implications. Hydrobiologia 361:89–99.
Sato, T. 1986. A brood parasitic catfish of mouthbrooding cichlid fishes in Lake Tanganyika. Nature 323:58–59.
Seegers, L. 1996. The fishes of the Lake Rukwa drainage. Musée Royal de l’Afrique Centrale, Annales, Sciences Zoologiques 278:1–407.
Seegers, L. 2008. The Catfishes of Africa. A handbook for identification and Maintenance. Aqualog Verlag, Rodgau, Germany.604 pp.
Stewart, K.M. 2001. The freshwater fish of Neogene Africa (Miocene-Pleistocene): Systematics and biogeography. Fish and Fisheries (Oxford) 2:177–230
Vigliotta, T.R. 2008. A phylogenetic study of the African catfish family Mochokidae (Osteichthyes, Ostariophysi, Siluriformes), with a key to genera. Proceedings of the Academy of Natural Sciences of Philadelphia 157:73–136.
Winemiller, K.O., and L.C. Kelso-Winemiller. 1996. Comparative ecology of catfishes of the Upper Zambezi River floodplain. Journal of Fish Biology 49:1043–1061.
Wisenden, B.D. 1999. Alloparental care in fishes. Reviews in Fish Biology and Fisheries 9:45–70.
Wright, J.J. and L.M. Page. 2006. Taxonomic revision of the Lake Tanganyikan Synodontis (Siluriformes: Mochokidae). The Bulletin of the Florida Museum of Natural History 46:99–154.
Wright, J.J. and L. M. Page. 2008. A new species of Synodontis (Siluriformes: Mochokidae) from tributaries of the Kasai River in northern Angola. Copeia 2008(2):294–300.
Title Illustrations

| Scientific Name | From left to right: (top) Synodontis katangae, Chiloglanis occidentalis, Atopochilus dybowskii, Microsynodontis emarginata; (middle) Chiloglanis kalambo, Euchilichthys royauxi, Acanthocleithron chapini, Mochokus niloticus; (bottom) Synodontis flavitaeniata, Mochokiella paynei, Synodontis ornatissima, Chiloglanis macropterus |
|---|---|
| Comments | various Mochokidae |
| Specimen Condition | Dead Specimen |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0.
|
| Copyright |
©

|
About This Page
John P. Friel

Cornell University Museum of Vertebrates, Ithaca, New York, USA

Cornell University Museum of Vertebrates, Ithaca, New York, USA
Correspondence regarding this page should be directed to John P. Friel at and Thomas R. Vigliotta at
Page copyright © 2009 John P. Friel and
 Page: Tree of Life
Mochokidae . African Squeaker and Suckermouth Catfishes.
Authored by
John P. Friel and Thomas R. Vigliotta.
The TEXT of this page is licensed under the
Creative Commons Attribution License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Mochokidae . African Squeaker and Suckermouth Catfishes.
Authored by
John P. Friel and Thomas R. Vigliotta.
The TEXT of this page is licensed under the
Creative Commons Attribution License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 02 March 2009
- Content changed 02 March 2009
Citing this page:
Friel, John P. and Thomas R. Vigliotta. 2009. Mochokidae . African Squeaker and Suckermouth Catfishes. Version 02 March 2009 (under construction). http://tolweb.org/Mochokidae/15214/2009.03.02 in The Tree of Life Web Project, http://tolweb.org/
 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
go to top
top
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
go to top
top