Gymnotiformes
The Neotropical Electric Eels and Knifefishes
James S. Albert and John G. Lundberg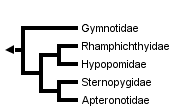

This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxNotes about Terminal Taxa
Electrophorus electricus, the electric eel, is here included in the family Gymnotidae to emphasize its close relationship with Gymnotus. Some authors give family status to the electric eel.
List of synapomorphies
Gymnotiformes.--27 characters unambiguously diagnose the Gymnotiformes, 17 of which are unique and unreversed among the otophysan fishes:
- no maxillary teeth
- maxillary head articulating with autopalatine cartilage
- lateral ethmoid reduced, margins not contacting other bones of neurocranium (except Rhamphichthyidae, Archolaemus, some species of Sternopygus and Magosternarchus)
- lateral margins of parasphenoid not extending to a horizontal with trigeminal foramen
- three derived features of telencephalic area Dorsalis (Albert and Lannoo, submitted)
- subcutaneous eye
- no accessory optic tract and nucleus (except sternopygids)
- no taste buds on extraoral integument
- no schreckstoff and fright response
- suite of characters associated with active, high frequency electroreception (Carr and Maler, 1986)
- tuberous-shaped high frequency electroreceptor organs
- autopalatines unossified
- no ectopterygoid
- no mesopterygoid teeth (present in Sternopygidae excluding Distocyclus, and present in Platyurosternarchus)
- gill rakers reduced, not contacting gill bar (except Electrophorus, Sternopygus, Rhabdolichops, and Sternarchella)
- opercle triangular
- ascending ramus of fifth epibranchial elongate, contacting fourth epibranchial
- no pelvic fins or girdle
- no claustrum
- capacity to regenerate postcoelomic axial structures
- no dorsal or adipose fins
- anal fin long, with more than 150 rays
- anal-fin rays articulate with proximal anal-fin pterygiophores
- cartilaginous hypural-opisthural rod (except Apteronotidae)
- anus located anterior to midlength of body
- hypaxial electric organs (replaced during metamorphosis in Apteronotidae and Sternopygidae)
- jamming avoidance response (except Sternopygus)
(James S. Albert)
Discussion of Phylogenetic Relationships
This discussion presents synapomorphies for higher level relationships among gymnotiform clades.
Gymnotidae.--14 characters support Ellis's (1913) hypothesis that Gymnotus and Electrophorus form a natural taxon which is the sisterlineage to other gymnotoids (the Gymnotoidei of Mago-Leccia, 1978,1994); large gape; premaxilla large, articulating head of maxilla oriented anteriorly; lateral margin of premaxilla produced into a posterior process; ventral margin of descending maxillary blade ith a sharp angle about two-thirds distance to its tip; base oflateral ethmoid narrow; cranial fontanels closed; m. adductor mandibulae undivided bundle at origin; lateral valvula of cerebellum large; depression on dorsal surface of basihyal; ventral process of coracoid absent; anal-fin pterygiophores long,more than one-third total body depth; caudal fin or filament absent, caudal anal-fin rays extend to tip of tail; body cavity long, more than 30 precaudal vertebrae; posterior chamber of gasbladder elongate, passing between hemal arches of postcoelomic axial skeleton and musculature.
Sternopygoidea.--This taxon was proposed by Mago-Leccia (1978) to include Rhamphichthyidae, Hypopomidae, Sternopygidae, andApteronotidae. Five characters support the monophyly of thisclade; branchial opening small; sagittal orientation of maxilla; anterior tip of mesethmoid small; displaced hemal spines; allometric growth of anus). An alternative hypothesis of relationships, uniting the Rhamphichthyoidea with the Gymnotoidea is supported by twocharacters unique to these taxa; morphologically distinct P and T type tuberous receptor organs; no distal anal-fin pterygiophores. These characters may be interpreted to be present in plesiomorphic gymnotoids and reversed in the Sternopygidae and Apteronotidae. The polarity of the pulse type EOD and associated neural characters is rendered ambiguous under this hypothesis of relationships. The phylogenetic distribution of the ascending process of the mesopterygoid also depends on relationships among basal gymnotoid taxa.
Rhamphichthyoidea.--The monophyly of the clade composed of Rhamphichthyidae + Hypopomidae is well corroborated; 18 diagnostic characters are reported by Albert (submitted).
Sternopygidae + Apteronotidae.--The monophyly of this taxon is supported by eight characters; posterior lobe of EGp well developed, extending to posterior margin of ELL; several (1-3)small fenestrae in lateral wall of neural arches; size and shape of anterior displaced hemal spine; ontogenetic replacement of larval hypaxial electric organ with adult organ; high frequency wave-form electric organ discharge (EOD) and associated neural substrates (205); EOD frequency shift during development); capacity to swim forwards and backwards, detecting prey objects by scanning across longitudinal electroreceptor array. Although members of these two taxa generate wave type EODs by different mechanismsm, wave form gymnotoids share a high repetition rate in which individual pulses overlap to form a quasi-sinusoidal discharge pattern. In species exhibiting the pulse type EOD,individual EODs are produced at low frequencies as non-overlapping pulses.
An additional four characters of ambiguous optimization further distinguish members of Sternopygidae + Apteronotidae. The anterior process of the maxilla is hook-shaped in all sternopygids,except Rhabdolichops in which the presence of an ossified laminar ridge obscures the morphology of this region. A similar morphology is observed in juvenile specimens of all members of the Apteronotinae except the Sternarchella and an undescribed genus being described by Lundberg et. al. Of the gymnotoid species examined, only sternopygids and apteronotids possess fasciculated laterosensory afferents, but no rhamphichthyoid taxon has yet been assessed (Lannoo and Maler,1990). Also, there is no mesocoracoid in Sternopygidae + Apteronotidae except Sternarchorhamphus and Sternopygus aequilabiatus, and the anal-fin extends anterior to the base of the pectoral-fin (also in Rhamphichthyidae).
Alternative hypotheses.--The branching order of the major lineages presented by Triques (1993, fig. 24) and Gayet et al. (1994, fig.11-A) are similar in the position of Apteronotidae as the sister taxon to other gymnotoids, and in placing Sternopygidae as the sister taxon to a clade consisting of Gymnotoidea and Rhamphichthyoidea (Alves-Gomes et al., 1995, fig. 2-B). The topology preferred by Alves-Gomes et al., (1995) concurs with the topolgy provided here in uniting the taxa possessing a high frequency, wave-form EOD (Apteronotidae and Eigenmanninae), and in placing Gymnotoidea as a relatively basal member of the Gymnotiformes.
Electrophysiology and behavior
The American knifefishes (Teleostei: Gymnotiformes) are animportant component of the nocturnal ichthyofauna of Middle and South American freshwaters. Over 100 gymnotoid species have beendiscovered in the neotropics, ranging from Río de La Plata of Argentina (35 degrees S) to the Río San Nicolas of southwestern Chiapas,Mexico (18 degrees N). In addition, many undescribed species are recognized in museum collections, and more forms are discovered each year.
An outstanding feature of gymnotoid biology, observed in allknown species, is the capacity to produce and detect weak electricfields. Gymnotoid fishes continually emit electric discharges,which they use in object location and communication by detecting changes in the shape, amplitude, or frequency of the electricfield. This electrosensory system has been the subject of numerous physiological, anatomical, and behavioral studies. Several review volumes have been published in recent years (Bullock andHeiligenberg, 1986; Heiligenberg, 1991) summarizing the major discoveries of this research program.
The sensory components of this system include the integumental hair cell receptors, as well as their peripheral and central nervous system connections. These structures are very similar to those described in other electroreceptive vertebrate groups despite the fact that electroreception appears to have evolved several times independently. Many of the similarities shared by electroreceptive lateral line systems are in fact also observed in the mechanosensory lateral line system of craniates, indicating their derivation from a common developmental pathway. Gymnotoids are also characterized by the presence of anelongate anal fin, with more than 200 anal-fin rays, which givesthem a knife-like appearance. This feature is functionally coupledwith electroreception and is the source of their common names inmany languages, including "knifefish" in English, and "cuchillo"(knife) or "anguilla" (eel) in Spanish. Gymnotoid fishes locomote by undulating this elongate anal fin, rather than the lateral body-wall musculature as do other fishes. Since the entire surface of gymnotoids is covered by electroreceptor organs, maintaining a somewhat rigid body axis facilitates the use of the integument asan undistorted sensory surface. The shimmering appearance of the undulating anal fin, and the nocturnal habits of knifefishes, arethe origin of the name "sarapo" (ghost) used by the Tupi-Guarani indigenous Amazonian peoples.
The motor effectors of the electrosensory system are specialized muscle or nerve cells called electrocytes which generate rhythmic electric organ discharges (EOD). Certain aspects of the frequency spectrum and wave form of the EOD appear to beunique to each species while other aspects may be used to characterize entire gymnotoid linages. Electric organ discharges are also used as social signals, in both territorial and sexual behaviors.
Electric fishes have been informally classified as either"wave" or "pulse" species depending on the EOD repetition rate. The relative ease with which these EOD signals and electroreceptor responses may be monitored has facilitated the use of gymnotoids in behavioral studies. Data collected in the field and laboratory settings confirm the role of electroreception in both trophic and social interactions. Investigations into the neural basis of electroreception has also provoked interest into methods of breeding and raising gymnotoids in captivity. As a result, comparative data are now available on what would be otherwise inaccessible character systems, including reproductive behavior,the behavior of larvae and juveniles and patterns in the development of the electric organs. The ability to breed knifefish in captivity has also permitted the application of experimental methods to the study gymnotoid regeneration. These advances facilitated the recognition of a fossil gymnotoid, Ellisella kirschbaumi, from the Upper Miocene (c. 10-12 mya) of Bolivia.
References
Albert, J.S. Phylogenetic systematics of the American knifefishes (Teleostei: Gymnotoidei). Misc. Publ. Mus. Zool., Univ. Michigan. submitted.
Alves-Gomes, J.A, G. Ortí, M. Haygood, A. Meyer, W. Heiligenberg. 1993. Phylogenetic analysis of the South American electric fishes (Order Gymnotiformes) and the evolution of their electrogenic system: A synthesis based on morphology, electrophysiology, and mitochondrial sequence data. Mol. Biol. Evol. 12(2):298-318.
Bullock, T.H. and W. Heiligenberg. 1986. Electroreception. Wiley- Interscience, New York. 722pp. Chapters by A.H. Bass (Electric organs revisited, pp. 13-70), J. Bastian (Electrolocation: behavior, anatomy, physiology, pp. 577-612), and C.E. Carr and L. Maler (Electroreception in gymnotiform fish: central anatomy and physiology, pp. 319-374).
Ellis, M.M. 1913. The gymnotid eels of tropical America. Mem. Carneg. Mus. 6(3):109-195.
Gayet, M., F.J. Meunier, and F. Kirschbaum. 1994. Ellisella kirschbaumi Gayet & Meunier, 1991, gymnotiforme fossile de Bolivie et ses relations phylogenetiques au sein des formes actuelles. Cybium 18(3):273-306.
Heiligenberg, W.F. 1991. Neural Nets in Electric Fish. MIT Press, Cambridge.
Lundberg, J.G., W.M. Lewis, J.F. Saunders, and F. Mago-Leccia. 1987. A major food webb component in the Orinoco River channel: evidence from planktivorous electric fishes. Science 237:81-83.
Mago-Leccia, F. 1994. Electric Fishes of the Continental Waters of America. Biblioteca de la Academia de Ciencias Fisicas, Matematicas, y Naturales, Caracas, Venezuela 29:1-206.
Rasnow, B. 1994. The Electric Field of a Weakly Electric Fish. Ph.D. Thesis, California Institute of Technology, Pasadena, California, 139 pp.
Triques, M.L. 1993. Filogenia dos generos de Gymnotiformes (Actinopteryigii, Ostariophysi), com base em characters esqueleticos. Comun. Mus. Ciene. PURCS, ser. zool. Porto Alegre 6:85-130.
Zakon, H.H. 1986. The electroreceptive periphery. Pp. 103-156 in Electroreception (T.H. Bullock and W. Heiligenberg, eds.). Wiley-Interscience, New York.
Title Illustrations

| Scientific Name | Electrophorus electricus |
|---|---|
| Location | Orinoco Delta |
| Comments | Head of the intriguing electric eel |
| Acknowledgements | Photo taken during the R/V Eastward expedition to the Orinoco Delta, 1979. |
| Body Part | head |
| Copyright | © 1979 Don Stewart |
About This Page
James S. Albert

University of Louisiana, Lafayette, Louisiana, USA
John G. Lundberg

The Academy of Natural Sciences, Philadelphia, Pennsylvania, USA
Page copyright © 1995 James S. Albert and John G. Lundberg
 Page: Tree of Life
Gymnotiformes. The Neotropical Electric Eels and Knifefishes.
Authored by
James S. Albert and John G. Lundberg.
The TEXT of this page is licensed under the
Creative Commons Attribution License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Gymnotiformes. The Neotropical Electric Eels and Knifefishes.
Authored by
James S. Albert and John G. Lundberg.
The TEXT of this page is licensed under the
Creative Commons Attribution License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Citing this page:
Albert, James S. and John G. Lundberg. 1995. Gymnotiformes. The Neotropical Electric Eels and Knifefishes. Version 01 January 1995 (under construction). http://tolweb.org/Gymnotiformes/15064/1995.01.01 in The Tree of Life Web Project, http://tolweb.org/








 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site