Polypodiidae
Leptosporangiate Ferns
Carl Rothfels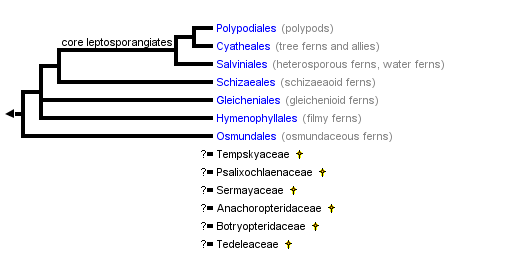

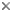
This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxRelationships after Smith et al. 2006
Introduction
Next to the flowering plants, the leptosporangiate ferns are the most diverse group of living land plants. Recent estimates place their diversity at about 12,000 species in 300 genera. The earliest known occurrence of leptosporangiate ferns is in the Lower Carboniferous. The five or more fern families recognized from this time were extinct by the Permian. Subsequent major filicalean evolutionary radiations during the Early Mesozoic yielded several families with extant representatives, but it was not until the Upper Cretaceous, after flowering plants had become dominant over much of the land surface, that much of extant fern diversity seems to have appeared.
Characteristics
(Extracted in large part from Wagner and Smith 1993)
Life Cycle
The fern life cycle is characterized by having two separate free-living plants, gametophytes and sporophytes, interconnected by stages of the sexual process. This phenomenon is referred to as an alternation of phases of a single generation. Ordinarily, the more conspicuous and dominant plant is the sporophyte (diploid and 2n), which is usually perennial and lives for an indefinite period. The gametophytes (haploid and n) tend to be inconspicuous and short-lived.
Organs - Roots, Stems, and Leaves
Roots of ferns tend to arise along the stem, commonly near leaf bases. They have been poorly surveyed, and our knowledge of them is limited, though recent work by Schneider (1996) has done much to improve this.
The stems of pteridophytes are mainly true rhizomes, i.e., stems that run horizontally at or just beneath the surface of the ground. Some ferns have upright stems like tiny tree trunks and are unable to form colonies except for occasional upright branchings. Rarely, a pteridophyte may have both upright stems and horizontal rhizomes. The rhizomatous habit of many ferns results in extensive vegetative reproduction. Anatomically the stems of pteridophytes are simpler than in most seed plants because they are made up only of primary tissues.
Fern leaves are often referred to as fronds. These develop in a distinctive manner with the soft growing tip (meristem) rolled up in the center of the crozier (fiddlehead). The crozier is produced by a process of growth and unfolding, and the characteristic pattern thus formed is known as circinate vernation. Leaf development that is initiated at the tip is called acropetal, and this development is characteristic of most modern ferns. Leaves of seed plants, in contrast, tend to develop by overall growth and expansion, i.e., by intercalary differentiation and enlargement.
The parts of a leaf include the petiole (stipe) and the blade (lamina). Petioles of ferns are diverse and offer many taxonomically valuable characteristics. The patterns are characteristic of genera and sometimes families. The main axis of the blade is called the midrib (rachis). If the blade of a fern is divided into leaflets, these are called pinnae, and if the leaflets are divided again into subleaflets, the subdivisions are called pinnules. If a blade has no subdivision it is termed simple; if it is once-divided only, it is regarded as 1-pinnate. If the pinnae are divided further into pinnules, the blade is 2-pinnate. A very finely divided blade may be 3 (or more)-pinnate (decompound).
Indument - Hairs, Scales, and Glands
In ferns, outgrowths of the epidermis of stems and leaves are common and varied, and these features are very important in description and identification. Simple outgrowths made up of a single cell or a chain of several cells are generally called hairs or trichomes. Trichomes that have two or three parallel rows of cells at the base and a single file of cells at the tip that are called bristles (setae). More elaborate outgrowths that form flat plates of 3-20 or more rows of cells are scales (paleae). Scales may be attached basally or centrally on a small stalk. One of the more distinctive features of many hairs and scales is the presence of enlarged and rounded terminal secretory cells. Glandular hairs, sometimes called glands, may characterize particular species. Such glands are not to be confused with nectaries, which are also secretory structures. Nectaries are rare in ferns and have been found in bracken fern and certain polypodies. Gametophytes of most ferns tend to have distinctive brownish or colorless hairs (rhizoids) that apparently serve to collect water and to anchor the plant.
Veins - Free, Dichotomous, or Reticulate
Many ferns have pinnately arranged free veins, with the major veins running out from the rachis laterally and the minor veins repeating the pattern. In leaves in which the veins come together to form a network of loops or meshes (veins anastomosing; venation reticulate), the area within a loop is an areole. Rarely, the venation of a pinna or pinnule may be entirely dichotomous with the veinlets repeatedly forked from the base to the tip of the segments. One of the special features of truly dichotomous venation is that there is no midrib. The small, juvenile leaves of many ferns have dichotomous venation, but distinct midribs are generally found in more adult, larger leaves.


Sporangia grouped together in roundish sori and covered by protective kidney-shaped indusium
Sporangia
The spore-producing organ of ferns is the sporangium. With few exceptions, sporangia are borne on leaves or modified leaves. When the leaves that bear sporangia are like those that are only photosynthetic, the leaves are called monomorphic. If, however, the sporangia are borne on leaves or leaflets that are strongly modified and different from the photosynthetic leaves, the leaves are said to be dimorphic. Sporangia of all "fern allies" and eusporangiate ferns are basically like those of the pollen-producing microsporangia of gymnosperms or the anthers of angiosperms in having thick walls with a number of cell layers. The sporangia open usually by a transverse split and produce hundreds or thousands of spores. Most ferns, however, have drastically modified and reduced sporangia that are so simple, they appear to be little more than elaborate trichomes bearing a tiny round spore case at the top. This distinctive type is called the leptosporangium. The spore case itself is few-celled, and the outer wall is only one cell thick at maturity. The number of spores produced in leptosporangia is usually only 128, 64, or 32, most commonly 64. Most leptosporangia have a bow or annulus made up of strongly modified, thickened cells. The position and extent of the annulus are important characteristics that define a number of fern families. In ferns, sporangia are on leaves or leaf parts, in various arrangements, solitary to many, often in clusters (sori) that are linear, oval, or round. Sori are often protected by flaps formed by the leaf margin (false indusia) or specialized scales or cuplike coverings (true indusia) that are separately produced from the area on which the sorus is borne (receptacle).
Spores
Spores of most modern ferns are all of one type, and the taxa are said to be homosporous. Individual spores are mostly 20–60 micrometers in length or diameter. Spores may be tetrahedral or nearly globose, and the scar (laesura) on the inner (proximal) surface may be triradiate; or the spores may be more or less reniform or bean-shaped and bilateral, with a single straight laesura. The type with the triradiate laesura is called trilete, and the one with the linear laesura, monolete. Some ferns have evolved the condition known as heterospory, in which two types of spores are produced by a given species: small spores mostly 20–30 micrometers long (called male) and large spores 200–700 micrometers in diameter (called female).


Fern gametophyte with young developing sporophyte in notch
Gametophytes
Sexual fusion in ferns occurs on inconspicuous, free-living plants known as gametophytes. Most gametophytes are green and surficial (surface borne), but some are nongreen and subterranean. Both surficial and subterranean gametophytes depend on free water through which the sperm must swim to reach the egg and achieve fertilization. The male gametes (sperms) are provided with special organelles equipped with cilia that propel the gametes by their motion. Sperms are produced in specialized cases (antheridia) that are either sunken in the gametophyte or protrude from it. The antheridia release the sperms through a pore. Release depends on maturity of the sperms and the presence of water. The female gamete (egg) is located in a bottlelike organ, the archegonium. Sperms swim to the opening at the top of the neck of the archegonium. The neck provides a passageway to the enlarged base (venter), where the egg is located and where fertilization takes place. It is believed that most fern gametophytes pass sequentially through all-archegonial or all-antheridial stages before becoming bisexual. Female gametophytes release soluble substances (antheridiogens) that stimulate nearby gametophytes to develop antheridia only. This tends to promote cross-fertilization between gametophytes of the same or closely related taxa.
Chromosomes
Spore mother cells, located inside the developing sporangia, are often used to study the chromosomes of ferns. Homosporous ferns have high chromosome base numbers (i.e., the lowest haploid multiple) ranging from about x=20 to x=110. These are among the highest base numbers known in vascular plants. Heterosporous ferns have low chromosome base numbers like those of most seed plants, i.e., x=7-11. This generalization applies across all divisions of pteridophytes. Polyploidy may be superimposed on the base numbers and, at least among homosporous taxa, very high numbers are sometimes attained. By observing meiotic chromosomes, one can usually determine whether a given plant is a hybrid or not. Meiosis in hybrids is generally very irregular, and the spores that result are malformed.
Discussion of Phylogenetic Relationships
The relationships between the major leptosporangiate ferns are generally well-supported, based primarily on chloroplast DNA sequence data (Hasebe et al. 1994, 1995; Pryer et al. 1995, 2001, 2004; Schuettpelz and Pryer 2007; Schuettpelz et al. 2006; Smith et al. 2006). The topology presented here is that of Smith et al. 2006.
References
Galtier, J. and A.C. Scott. 1985. Diversification of early ferns. Proc. Roy.Soc. Edinb. 86B:289-301.
Gensel, P.G. 1992. Phylogenetic relationships of the zosterphylls and lycopsids: Evidence from morphology, paleoecology, and cladistic methods of inference. Ann. Missouri Bot. Gard. 79: 450-473.
Hasebe, M., T. Omori, M. Nakazawa, T. Sano, M. Kato, and K. Iwatsuki. 1994. rbcL gene sequences provide evidence for the evolutionary lineages of leptosporangiate ferns. Proc. Natl. Acad. Sci. USA 91: 5730-5734.
Hasebe, M., P.G. Wolf, K.M. Pryer, K. Ueda, M. Ito, R. Sano, G.J. Gastony, J. Yokoyama, J.R. Manhart, N. Murakami, E.H. Crane, C.H. Haufler, W.D. Hauk. 1995. Fern phylogeny based on rbcL nucleotide sequences. Amer. Fern J. 85: 134-181.
Holttum, R.E. 1947. A revised classification of leptosporangiate ferns. J. Linn. Soc. (Bot.) 53: 23-159.
Holttum, R.E. 1949. The classification of ferns. Biol. Rev. 24: 267-296.
Holttum, R.E. 1973. Posing the problems. In A.C. Jermy, J.A. Crabbe, and B.A. Thomas.(eds.), The phylogeny and classification of the ferns, pp. 1-10. Bot. J. Linn. Soc. 67 (Suppl. 1).
Kenrick, P. and P.R. Crane. 1997a. The origin and early evolution of plants on land. Nature: in press.
Kenrick, P. and Crane, P.R. 1997b. The origin and early diversification of land plants: a cladistic study. Smithsonian Press (in press).
Kranz, H. D. and V. A. R. Huss. 1996. Molecular evolution of pteridophytes and relationships to seed plants: evidence from complete 18S rRNA gene sequences. Plant Systematics and Evolution (in press).
Kranz, H.D., D. Miks, M. Siegler, I. Capesius, C.W. Sensen, and V.A.R. Huss. 1995. The origin of land plants: phylogenetic relationships among charophytes, bryophytes, and vascular plants inferred from complete small-subunit ribosomal RNA gene sequences. J. Mol. Evol. 41: 74-84.
Kubitzki, K. (ed.). 1990. The families and genera of vascular plants. Vol. 1. Pteridophytes and gymnosperms. K.U. Kramer and P.S. Green (vol. eds.). Springer-Verlag, Berlin.
Lovis, J.D. 1977. Evolutionary patterns and processes in ferns. In R.D. Preston and H.W. Woolhouse (eds.), Advances in botanical research, Vol. 4, pp. 229-415. Academic Press, London.
Manton, I. 1950. Problems of cytology and evolution in the Pteridophyta. Cambridge University Press, London.
Manhart, J.R. 1995. Chloroplast 16S rDNA sequences and phylogenetic relationships of fern allies and ferns. Amer. Fern J. 85: 182-192.
Mickel, J.T. 1974. Phyletic lines in the modern ferns. Ann. Missouri Bot. Gard. 61: 474-482.
Pichi Sermolli, R.E.G. 1977. Tentamen pteridophytorum genera in taxonomicum ordinem redigendi. Webbia 31: 313-512.
Pryer, K.M., A.R. Smith, and J.E. Skog. 1995. Phylogenetic relationships of extant ferns based on evidence from morphology and rbcL sequences. Amer. Fern J. 85: 205-282.
Pryer, K. M., H. Schneider, et al. 2001. Horsetails and ferns are a monophyletic group and the closest living relatives to seed plants. Nature 409: 618-622.
Pryer, K. M., E. Schuettpelz, et al. 2004. Phylogeny and evolution of ferns (monilophytes) with a focus on the early leptosporangiate divergences. Amer. J. Bot. 91: 1582-1598.
Raubeson, L.A. and D.B. Stein. 1995. Insights into fern evolution from mapping chloroplast genomes. Amer. Fern J. 85: 193-204.
Rothwell, G.W. 1987. Complex Paleozoic filicales in the evolutionary radiation of ferns. Amer. J. Bot. 74: 458-461.
Rothwell, G.W. 1994. Phylogenetic relationships among ferns and gymnosperms; an overview. J. Plant Res. 107: 411-416.
Rothwell, G.W. 1995. The fossil history of branching: Implications for the phylogeny of land plants. Pp 71-86 in: P.C. Hoch and A.G. Stevenson (eds.). Experimental and molecular approaches to Plant Biosystematics. Missouri Botancial Garden, St. Louis.
Rothwell, G.W. 1996. Pteridophytic evolution: an often underappreciated phytological success story. Rev. Palaeobot. Palynol. 90: 209-222.
Rothwell, G.W. and R.A. Stockey. 1994. The role of Hydropteris pinnata gen. et sp. nov. in reconstructing the cladistics of heterosporous ferns. Amer. J. Bot. 81: 479-492.
Schneider, H. 1996. Vergleichende Wurzelanatomie der Farne. Ph.D. Dissertation, Zurich University.
Schuettpelz, E., P. Korall, et al. 2006. Plastid atpA data provide improved support for deep relationships among ferns. Taxon 55: 897-906.
Schuettpelz, E. and K. M. Pryer. 2007. Fern phylogeny inferred from 400 leptosporangiate species and three plastid genes. Taxon 56: 1037-1050.
Smith, A.R. 1995. Non-molecular phylogenetic hypotheses for ferns. Amer. Fern J. 85: 104-122.
Smith, A. R., K. M. Pryer, et al. 2006. A classification for extant ferns. Taxon 55: 705-731.
Stewart, W.N. and G.W. Rothwell. 1993. Paleobotany and the evolution of plants, ed. 2. Cambridge University Press, Cambridge.
Taylor, T.N. and E.L. Taylor. 1993. The biology and evolution of fossil plants. Prentice-Hall, Inc., Englewood Cliffs, NJ.
Thomas, B.A. 1991. The study of fossil ferns. British Pteridology 1891-1991: 7-16.
Tryon, A.F. and B. Lugardon. 1991. Spores of the Pteridophyta. Springer-Verlag, New York.
Tryon, R.M. and A. Tryon. 1982. Ferns and allied plants, with special reference to tropical America. Springer-Verlag, New York.
Wagner, W. H., Jr. 1974a. Pteridology, 1947--1972. Ann. Missouri Bot. Gard. 61: 86--111.
Wagner, W. H., Jr. 1974b. Structure of spores in relation to fern phylogeny. Ann. Missouri Bot. Gard. 61: 332--353.
Wagner, W.H., Jr. and A.R. Smith. 1993. Pteridophytes. Pp. 247-266 in Flora of North America Editorial Committee (ed.). Flora of North America North of Mexico. Oxford University Press, New York.
Wolf, P.G. 1995. Phylogenetic analyses of rbcL and nuclear ribosomal RNA gene sequences in Dennstaedtiaceae. Amer. Fern J. 85: 306-327.
Wolf, P.G., P.S. Soltis, D.E. Soltis. 1994. Phylogenetic relationships of dennstaedtioid ferns: evidence from rbcL sequences. Mol. Phylog. Evol. 3: 383-392.
Wolf, P. G., K. M. Pryer, A. R. Smith, and M. Hasebe. in press. Phylogenetic studies of extant pteridophytes. In: Molecular Systematics of Plants, 2nd edition. P. S. Soltis, D. E. Soltis, J. J. Doyle (eds). Chapman and Hall, New York.
Title Illustrations

| Scientific Name | Melpomene moniliformis (Grammitidaceae) |
|---|---|
| Comments | Sporangium of the Bolivian fern Melpomene moniliformis (Grammitidaceae) showing the chlorophyllous spores (red), annulus (yellow), and stomium(blue). Autofluorescence image created using epifluorescence light illumination and a multichannel image capturing system. |
| Specimen Condition | Live Specimen |
| Body Part | leptosporangium |
| Copyright | © 2003 |
| Scientific Name | Sadleria cyatheoides Kaulf. |
|---|---|
| Location | Native to Hawaii (photo of plant in cultivation at Duke University) |
| Specimen Condition | Live Specimen |
| Copyright |
© 2004 Eric Schuettpelz

|
| Scientific Name | Cyatheales |
|---|---|
| Location | Costa Rica |
| Comments | tree fern |
| Specimen Condition | Live Specimen |
| Copyright | © 1967 William C. Burger |
About This Page
We are grateful to Brian R. Speer (Dept. of Integrative Biology, University of California at Berkeley); for his patient technical assistance.
Carl Rothfels

Duke University, Durham, North Carolina, USA
Correspondence regarding this page should be directed to Carl Rothfels at
Page copyright © 1997
All Rights Reserved.
- Content changed 23 December 2008
Citing this page:
Rothfels, Carl. 2008. Polypodiidae . Leptosporangiate Ferns. Version 23 December 2008 (under construction). http://tolweb.org/Polypodiidae/21666/2008.12.23 in The Tree of Life Web Project, http://tolweb.org/











 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site