Coccidia
Eucoccidiorida
Jan Šlapeta and Victoria Morin-Adeline

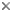
This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
Coccidia Leuckart, 1897 (syn. Eucoccidiorida Leger & Duboscq, 1910)
Coccidia are protozoan unicellular organisms of the phylum Apicomplexa that parasitise most major lineages of vertebrates as well as invertebrates (Fayer, 1980).
Due to taxonomical complications and the vast amount of data being compiled within the coccidia field, a specialist website has been erected in an attempt to record, store and and resolve taxonomical redundancy associated with these organisms. According to the “Coccidia of the World” compiled by Don Duszynski, Steve Upton and colleges, there are currently in excess of 2000 named species of coccidia distributed over 8-13 families. There is no doupt this is a true underestimation of the real diversity of coccidia, and many thousands remain undescribed.
Coccidian parasites are responsible for several of the most severe diseases known in animals and man (Levine, 1973). For example in domestic animals, Eimeria tenella is responsible for considerable decrease in growth and development of domestic poultry flocks by damage caused to the intestinal lining during infection, and costs to the industry exceed US$1.5 billion annually (Sharman et al., 2010).
Characteristics
Coccidia are complex unicellular parasites of vertebrates and invertebrates. They parasitise their host intracellularly. They are classified according to the following characteristics:
- Possession of an environmentally resistant oocyst wall (Lee et al., 2000). Sporozoites develop within a secondary cyst within the oocyst known as a sporocysts, with the exception of Cryptosporidum where they develop directly within the oocyst (Lee et al., 2000, Robinson et al., 2010)
- The number of sporocytes within an oocyst and the number of developing sporozoites within a sporocyte is genus dependant (Fayer, 1980). Eimeriid coccidians contain a steida body at the anterior pole of the sporocyst and a refractile body within sporozoites, while these features are not present within isosporoid coccidia (Barta, 2001)
- Sporozoites and merozoites are surrounded by a tri-layered pellicule. The outer membrane is continuous over the entire parasite, whereas the inner membranes are disrupted due to the presence of a micropore towards the anterior end (D'Haese et al., 1977)
- Sporozoites and merozoites contain an apical complex anterior to the cell, which contains a conoid structure made of tublin fibres (Hu et al., 2002, Lee et al., 2000)
- Possession of two polar rings within the apical complex (D'Haese et al., 1977).
Microtubules exude from an outer polar ring but do not reach the posterior of the cell; their number is species dependent (D'Haese et al., 1977).
Hosts
The traditional coccidians (Eucoccidia) have been described in all classes of vertebrates such as in fish, reptiles, birds, and mammals including humans (Levine, 1973). However the second coccidians (Adeleorina) includes species associated with invertebrates as well as parasites of vertebrates. The latter group infects blood cells of vertebrates and is collectively known as "haemogregarines". Veterinary important parasites in the genus Haepatozoon belong to this group.
Parasites within the coccidian group can be either monoxenous, parasitising a single host throughout their lifecycle, or heteroxenous whereby the parasite will parasite multiple hosts (Fayer, 1980, Lee et al., 2000). For instance, species belonging to the genera Eimeria and Isospora are well known monoxenous parasites, while those of Toxoplasma make use of an intermediate host (Fayer, 1980). It is thought that coccidians that have heteroxenous lifecycles can have a wide range of intermediate hosts, but they commonly have a narrow range for the final host (Fayer, 1980).
General Lifecycle
Monoxenous lifecycle
The most depicted of the monoxenous coccidian lifecycles is that of the geneus Eimeria in poultry, however others such as that of the genus Isospora share similar features (Fayer, 1980, Levine, 1973). An infection is established upon the ingestion of sporulated oocysts from contaminated feed or water by a vertebrate host and once inside the vertebrate stomach, the oocyst wall is broken down by mechanical and chemical action releasing the sporocytes which contain infective sporozoites (Lee et al., 2000, Levine, 1973). Excystation results in the opening of the anterior cap of sporocysts to release infective sporozoites. Interactions of the apical complex with the plasma membrane of epithelial cells allow sporozoites to penetrate the host cell (Grimwood and Smith, 1996). Within the cells, the sporozoites transform into merozoites which multiply asexually for 2-7 generations, the exact number being species dependent (Lee et al., 2000). With each asexual generation, the host cell is destroyed to release merozoites that infect new cells (Levine, 1973).

Developing oocysts of Eimeria tenella in the caecum of chicken causing serious coccidiosis. © Jan Slapeta
Following the final asexual division, the merozoites enter new epithelial cells and transform into gamonts, with the majority developing into macrogamtes (i.e female gametes), while a few undergo further division and develop flagella to become microgametes (i.e male gametes) (Fayer, 1980). The resultant diploid zygote produced upon fertilisation lays down a heavy resistant oocyst wall around itself and enters the intestinal lumen (Fayer, 1980). Sporulation occurs within the young oocyst to produce sporocysts, within which the infective sporozoites develop and contaminate the environment to begin a new cycle (Lee et al., 2000, Levine, 1973).
Heteroxenous lifecycle
Coccidians that display a heteroxenous lifecycle such as Toxoplasma gondii are transmitted between hosts by utilising the predator and prey relationship; that is, infective stages are produced within the prey (intermediate hosts) and the lifecycle is completed within the predator (definitive host) upon prey consumption. Briefly, the intermediate hosts for Toxoplasma gondii can be a wide range of unspecified animals and are responsible for harbouring tissue cysts resulting from the asexual reproduction stage (Innes, 2010).


Toxoplasma gondii tissue cyst in a mouse brain. © Jan Slapeta
Two stages of the parasites can be found within the cysts; the tachyzoite and bradyzoite stages, the former being a mutipication stage and the latter being the infective stage (Innes, 2010). Bradyzoites are released in the definitive host stomach upon ingestion of tissue cysts and they penetrate the intestinal epithelium (Lee et al., 2000). Within the intestinal epithelial cells, five generations of asexual reproduction occur which eventually end with the formation of gamonts (Lee et al., 2000). The gamonts eventually transform into macro (female) and micro (male) gametes and fertilise to form a zygote surrounded by an oocyst wall (Lee et al., 2000, Levine, 1973). Therefore stages within the definitive host intestines resemble that described for monoxenous coccidia above (Levine, 1973). The oocytes are passed though the host feces into the environment, where they are ingested by another intermediate hosts to begin the cycle again.
Jargon in Apicomplexa: tachyzoite, bradyzoite, merozoite and sporozoite
A number of term describing different life forms is used by parasitologists and molecular biologist studying transition from one stage to another. The motivation to study this transition is to identify and block such machinery and therefore kill the parasite or minimise the parasite's impact on its host. The table below summarises ultrastructural features of different stages of Toxoplasma gondii. Tachyzoites and bradyzoites are extraintestinal stages. The tachyzoite is the rapidly dividing infective stage often asociated wth acute disease - toxoplasmosis. The badyzoite is the slowly dividing stage that fills the tissue cysts. Merozoites and sporozoites are intestinal stages. Merozoites are invasive stages formed during a phase of rapid intestinal asexual multiplication. Sporozoites are formed at the end of the sexual phase of coccidian reproduction. Sporozoites are those long surviving stages inside the oocysts, therefore they contain polysaccharide granules that provide them with energy during the time of inactivity and during initial invasion once ingested by a new host. Besides morphologial dissimilarities these stages are differentiated by the presence of surface molecules, e.g. GRA, MIC, RON etc.
| Asexual stage | Nucleus | Rhoptries | Micronemes | Dense granules | Polysacharide granules |
|---|---|---|---|---|---|
| Tachyzoite | centrally | up to 12 | few | many | few |
| Bradyzoite | distal end | up to 10 | many | many | many |
| Merozoite | centrally | up to 5 | few | few | none |
| Sporozoite | distal end | up to 10 | many | many | many |
Classification of Coccidia
Mature gamonts intracellular, small; conoid not modified into mucron or epimerite; syzygy generally absent (if present involves gametes); sexual stages generally very different; different number of male and female gametes; microgametes without flagella; zygotes form oocysts from fertilised macrogametocyte; lifecycle consists of merogony, gametogony and sporogony; most species in vertebrates; homoxenous and heteroxenous; about 3000 named species.
Traditionally, Coccidia include additional odd parasites from marine invertebrates classified into separate classes Protococcidiorida (syn. Coelotrophida) or Agamococcidiorida based on absence of merogony and absence of both merogony and gamonts, respectively (these are extremely obscure species).
Suborder: Adeleorina Leger, 1911
Macrogamete and microgamete usually associated in syzygy; microgamont produces small number (1-4) of microgametes; sporozoites enclosed in oocyst; endodyogeny absent; homoxenous or heteroxenous; about 500 named species.
- Family Adeleidae Mesnil, 1903
- Family Hepatozoidae Wenyon, 1926 (~50 species)
- Family Klossiellidae Smith and Johnson, 1902
- Family Haemogregarinidae Leger, 1911 (~350 species)
Suborder: Eimeriorina Leger, 1911
Macrogamete and microgamete develop independently; syzygy absent; microgamont produces large mumber of microgametes; zygote not motile; sporozoites enclosed in sporocyst; endodyogeny absent or present; homoxenous or heteroxenous; about 2500 named species.
- Family Eimeriidae Minchin, 1903 (>2000 species)
- Family Sarcocystidae Poche, 1913 (>350 species)
- Family Aggregatidae Labee, 1899 (~25 species)
- Family Lankesterellidae Noller, 1902 (~25 species)
The suborder Eimeriorina Leger, 1911 to which the majority of coccidia parasites belong consists of up to eight families (Lee et al., 2000), four are mentioned above. Together with the smaller suborder Adeleorina (Leger, 1911), they make up the order Eucoccidiorida (Leger and Duboscq, 1910) which are a collective group of coccidians known to parasitise vertebrates and invertebrates (Lee et al., 2000). The suborder represents the majority of coccidia parasites of human and veterinary importance (Barta, 2001), which includes the families Eimeriidae and Sarcocystidae (Lee et al., 2000). We do not classify Cryptosporidiidae within Coccidia contrary to Lee et al. (2000). Cryptosporidium spp. are known to be a unique apicomplexan group that likely emerged within gregarines or independently.
Discussion of Phylogenetic Relationships
As with the rest of the phylum Apicomplexa, classification within the coccidian groups is largely a 'mess' due to incorrect or misinterpreted morphological data gathered over the years (Morrison, 2009, Long and Joyner, 1984). Increasingly, clade support is being sought to characterise parasites with the advent of molecular biology (Clopton, 2009). Due to the escalating amount of information being made available on the apicomplexans, such standardisation is vital to enable arrangement of this information for further use. For instance, molecular data have supported the use of sporocyst wall structure and presence of a refracile body within sporozoites as cardinal characters for a clade of coccidian parasites within the suborder Eimeriorina (Barta, 2001, Jirku et al., 2002). Furthermore, with the high turnover of coccidian species being named, genetic support is being advocated (Jirku et al., 2008). Even so, much of coccidian taxonomy remains problematic due to issues associated with erroneous/incomplete taxon sampling and use of bioinformatics tools (Morrison and Ellis, 1997, Morrison, 2009). The only group that has good coverage using molecular markers is the family Sarcocystidae. For the family Eimeriidae, too few genera were sequenced to reliably resolve the phylogeny; the majority of those sequenced are species of veterinary importance especially in the genus Eimeria.
 Hypothetical tree to illustrate unknow relationiship of the many genera formally named, only the yellow part is based on SSU rDNA including sequences of Lankesterella that branches withi Eimeridae dispite formally within its own family. © Jan Slapeta |  Tree based on SSU/LSU rDNA showing a sister relationiship between the two subfamilies (Sarcocystinae: g. Sarcocystis; Toxoplasmatinae: g. Toxoplasma, Neospora, Hammondia, Cystoisospora, Besnoitia and Hyaloklossia) © Jan Slapeta |
References
Barta, J. R. 2001. Molecular approaches for inferring evolutionary relationships among protistan parasites. Veterinary Parasitology, 101, 175-186.
Brooks, C. F., Johnsen, H., van Dooren, G. G., Muthalagi, M., Lin, S. S., Bohne, W., Fischer, K. & Striepen, B. 2010. The Toxoplasma Apicoplast Phosphate Translocator Links Cytosolic and Apicoplast Metabolism and Is Essential for Parasite Survival. Cell Host & Microbe, 7, 62-73.
Clopton, R. E. 2009. Phylogenetic relationships, evolution, and systematic revision of the septate gregarines (Apicomplexa: Eugregarinorida: Septatorina). Comparable Parasitology, 76, 167-190.
D'Haese, J., Mehlhorn, H. & Peters, W. 1977. Comparatiove electron microscope study of pellicular structures in coccidia (Sarcocustis, Besnoitia and Eimeria). International Journal for Parasitology, 7, 505-518.
Dolnik, O. V., Palinauskas, V. & Bensch, S. 2009. Individual oocysts of Isospora (Apicomplexa: coccidia) parasites from avian feces: from photo to sequence. Journal of Parasitology, 95, 169-174.
Fayer, R. 1980. Epidemiology of protozoan infections- Coccidia Veterinary Parasitology, 6, 75-103.
Grimwood, J. & Smith, J. E. 1996. Toxoplasma gondii: The role of parasite surface and secreted proteins in host cell invasion. International Journal for Parasitology, 26, 169-173.
Hijjawi, N. S., Meloni, B. P., Ng'anzo, M., Ryan, U. M., Olson, M. E., Cox, P. T., Monis, P. T. & Thompson, R. C. A. 2004. Complete development of Cryptosporidium parvum in host cell-free culture. International Journal for Parasitology, 34, 769-777.
Hu, K., Roos, D. S. & Murray, J. M. 2002. A novel polymer of tubulin forms the conoid of Toxoplasma gondii. Journal of Cell Biology, 156, 1039-1050.
Innes, E. A. 2010. A brief histroy and overview of Toxoplasma gondii. Zoonoses and Public Health, 57, 1-7.
Jirku, M., Modry, D., Slapeta, J., Koudela, B. & Lukes, J. 2002. The phylogeny of Goussia and Choleoeimeria (Apicomplexa; Eimeriorina) and the evolution of excystation structures in coccidia. Protist, 153, 379-390.
Jirku, M., Valigurova, A., Koudela, B., Krizek, J., Modry, D. & Slapeta, J. 2008. New species of Cryptosporidium Tyzzer, 1907 (Apicomplexa) from amphibian host: morphology, biology and phylogeny. Folia Parasitologica, 55, 81-94.
Leander, B. S., Lloyd, S. A. J., Marshall, W. & Landers, S. C. 2006. Phylogeny of marine gregarines (Apicomplexa) - Pterospora, Lithopystis and Lankesteria - and the origin(s) of coelomic parasitism. Protist, 157, 45-60.
Lee, J. J., Leedale, G. F. & Bradbury, P. (eds.) 2000. An illustrated guide to the Protozoa, Kansas: Society of Protozoologists.
Lei, C., Rider Jr. S, D., Wang, C., Zhang, H., Tan, X. & Zhu, G. 2010. The apicomplexan Cryptosporidium parvum possesses a single mitochondrial-type ferredoxin and ferredoxin: NADP+ reductase system. Protein Science, 19, 2073-2084.
Levine, N. D. 1973. The Apicomplexa and the coccidia proper. Protozoan parasites of domestic animals and man. 2nd ed. Minneapolis: Burgress Publishing Company.
Levine, N. D. 1982. Isospora passeris n. sp from the house sparrow Passer domesticus, Isospora lacazei, and related apicomplexan protozoa Transactions of the American Microscopical Society, 101, 66-74.
Long, P. L. & Joyner, L. P. 1984. Problems in the identification of species of Eimeria Journal of Protozoology, 31, 535-541.
Morrison, D. A. 2009. Evolution of the Apicomplexa: where are we now? Trends in Parasitology, 25, 375-382.
Morrison, D. A. & Ellis, J. T. 1997. Effects of nucleotide sequence alignment on phylogeny estimation: A case study of 18s rDNAs of Apicomplexa. Molecular Biology and Evolution, 14, 428-441.
Ralph, S. A., van Dooren, G. G., Waller, R. F., Crawford, M. J., Fraunholz, M. J., Foth, B. J., Tonkin, C. J., Roos, D. S. & McFadden, G. I. 2004. Metabolic maps and functions of the Plasmodium falciparum apicoplast. Nature Reviews Microbiology, 2, 203-216.
Robinson, G., Wright, S., Elwin, K., Hadfield, S. J., Sharman, P. A., Smith, N. C., Wallach, M. G. & Katrib, M. 2010. Chasing the golden egg: vaccination against poultry coccidiosis. Parasite Immunology, 32, 590-598.
Title Illustrations

| Scientific Name | Eimeria maxima |
|---|---|
| Comments | Oocyst of Eimeria maxima (Coccidia: Apicomplexa) |
| Specimen Condition | Live Specimen |
| Life Cycle Stage | oocysts |
| Image Use |
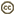 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0.
|
| Copyright |
© Jan Šlapeta

|
| Scientific Name | Eimeria maxima |
|---|---|
| Comments | Sporulated Eimeria maxima (Coccidia: Apicomplexa) |
| Specimen Condition | Live Specimen |
| Image Use |
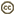 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0.
|
| Copyright |
© Jan Šlapeta

|
| Scientific Name | Hepatozoon ayorgbor |
|---|---|
| Identified By | Michal Sloboda |
| Life Cycle Stage | gametocytes |
| Body Part | erythrocytes of infected snakes |
| Copyright | © 11.3.2009 |
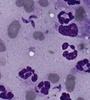
| Scientific Name | Toxoplasma gondii |
|---|---|
| Comments | Cat BAL with Toxoplasma gondii tachyzoites |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0.
|
| Copyright |
© Jan Šlapeta

|
About This Page
This page is being developed as part of the Tree of Life Web Project Protist Diversity Workshop, co-sponsored by the Canadian Institute for Advanced Research (CIFAR) program in Integrated Microbial Biodiversity and the Tula Foundation.
Jan Šlapeta

Faculty of Veterinary Science, University of Sydney, New South Wales, Australia
Victoria Morin-Adeline

Faculty of Veterinary Science, University of Sydney, Australia
Correspondence regarding this page should be directed to Jan Šlapeta at
Page copyright © 2011 Jan Šlapeta and Victoria Morin-Adeline
 Page: Tree of Life
Coccidia . Eucoccidiorida .
Authored by
Jan Šlapeta and Victoria Morin-Adeline.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Coccidia . Eucoccidiorida .
Authored by
Jan Šlapeta and Victoria Morin-Adeline.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 18 May 2011
- Content changed 18 May 2011
Citing this page:
Šlapeta, Jan and Victoria Morin-Adeline. 2011. Coccidia . Eucoccidiorida . Version 18 May 2011 (under construction). http://tolweb.org/Coccidia/124807/2011.05.18 in The Tree of Life Web Project, http://tolweb.org/




.200a.png)
.200a.png)


.100a.png)
.100a.png)

 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site