Acanthaceae
Lucinda A. McDade, Carrie Kiel, and Erin Tripp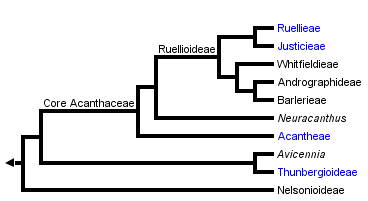

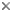
This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
The large and largely tropical plant family Acanthaceae includes at least 4000 species. The group is part of the order Lamiales which includes plants such as the familiar snap dragon (Plantaginaceae), mint (Lamiaceae) and African violet (Gesneriaceae). These plants have sympetalous corollas (i.e., with petals fused into a tube) and four stamens (sometimes reduced further to two).
Acanthaceae includes the 221 genera treated by Scotland & Vollesen (2000) plus the black mangrove genus, Avicennia. Notably, as a result of the largely tropical distribution of Acanthaceae, species-level diversity remains poorly understood, and there is little doubt that many new species remain to be discovered. In particular, the Neotropics house the richest and most poorly documented angiosperm flora on Earth. It is thus not surprising that Neotropical acanths remain incompletely known, and we expect many more species to be described from this region. As a quantitative indication of the pace of new discovery in Acanthaceae, Index Kewensis reports 81 new species described in the genus Justicia alone between 1986 and 1995. We expect the species count eventually to exceed 4000.
Characteristics
Plants belonging to the Core Acanthaceae (i.e., Acantheae through Ruellieae as indicated in the phylogeny presented at the top of this page; Acanthoideae sensu Scotland & Vollesen 2001) may be recognized by their fruit: a few-seeded, explosively dehiscent capsule within which seeds are borne on hook-like structures called retinacula (the lignified derivatives of the funiculus). These retinacula are a unique and unreversed synapomorphy for Acanthoideae: among these plants–all 4000 of them–fruits vary in size and in seed number but are otherwise remarkably homogeneous. The hooks presumably serve to propel the seeds away from the parent plant when the fruit dehisces but studies are lacking.


Retinacula of the fruit of Asystasia gangetica. © 2006 Gerald Carr
Within Core Acanthaceae, Acantheae are well marked as monophyletic by morphological and molecular synapomorphies. Its sister group which we have here called Ruellioideae is also marked by a number of morphological synapomorphies including cystoliths (crystals) in most plant structures.

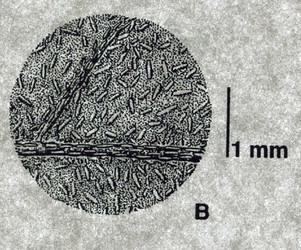
Cystoliths are the small linear structures that 'dot' the lower surface of this leaf of a South American Ruellia species. © C. Ezcurra
In addition to this core group, four other small lineages are very closely related and are now treated as part of a broad Acanthaceae: the pantropical and subtropical black mangroves, Avicennia; Old World Thunbergia (including the commonly cultivated black-eyed Susan, Thunbergia alata) and related genera, Old and New World Mendoncia; and Nelsonioideae (Nelsonia, Elytraria, Staurogyne, a handful of other small genera). Each of these four groups has its own distinctive fruit type. Unfortunately, we have yet to identify a synapomorphy for all Acanthaceae that is as notable and easy to recognize as fruit type is for the core group. Compared to most other Lamiales, Acanthaceae have relatively few ovules.
Beyond fruits of Acanthaceae, diversity in other traits is spectacular. Plants are rosette and caulescent herbs, shrubs and trees (rarely vines except Thunbergia and Mendoncia which are almost entirely climbing plants). Most acanths have solid green leaves, but a few have remarkably colored leaves, and some are cultivated for their foliage (e.g., polka dot plant, Hypoestes phyllostashya; Persian shield, Strobilanthes dyerianus). Inflorescences of many acanths have large, colorful bracts that often eclipse the corollas in showiness (e.g., Pachystachys, Megaskepasma; see images below). However, bracts of plants of many other species are diminutive, green and clearly not involved in attracting pollinators. Many species have extremely showy flowers and a number are cultivated for their beauty (e.g., Justicia brandegeana and many other species belonging to this large genus, Aphelandra squarrosa; you will see examples below); however, many others have diminutive green bracts and very small flowers. Across the family, flowers range in size from a few mm to more than 10 cm, with a parallel range of variation in shape and color. Variation in these corolla traits is no doubt associated with a wide range of animal pollinators, although studies are remarkably few.




Pachystachys lutea Nees (left, © 2006 Lucinda A. McDade) and Megaskepasma erythrochlamys Lindau (middle, © 2003 Shigenobu Aoki Botanical Garden) have large, colorful bracts. Justicia betonica L. (right, © Gerald D. Carr) is cultivated because of its showy flowers.
Pollen and Chromosomes
Structural diversity continues below the visible level in Acanthaceae. In terms of number and type of apertures and, especially in terms of ultrasculpturing, pollen grains of Acanthaceae are without doubt as diverse as any other family-level lineage regardless of size.




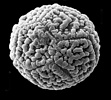

Pollen grains of Acanthaceae. From left to right: Crossandra sulphurea, © 1990 Carol Furness. Acanthus mollis, Acanthus spinosus, Neriacanthus grandiflorus, Aphelandra flammea, and Crossandra pungens, © T. F. Daniel.
Chromosome numbers are similarly diverse, with documented haploid numbers ranging from 7 to 68, thus indicating many evolutionary transitions via both dysploidy and polyploidy.
Discussion of Phylogenetic Relationships
There is at present little disagreement among students of Acanthaceae regarding membership in the family and delineation of the main clades as presented in the phylogeny above. There is, however, quite a bit of work to be done to resolve relationships at a number of levels. For example, the results of Schwarzbach & McDade (2001) placed Avicennia sister to Thunbergioideae but without strong support from molecular data for this relationship and with no identifiable morphological synapomorphies supporting the relationship. Similarly, the placement of Neuracanthus among the lineages of core Acanthaceae (i. e., the lineages with retinacula) have yet to be fully resolved.
There also remains a great deal of work to be done on relationships within lineages. At present (August 2006), we present intra-lineage phylogenetic information for Acantheae, for Isoglossinae and the Tetramerium Lineage within Justicieae, and for Ruellieae. Our continuing work and that of our colleagues will gradually add knowledge of relationships within the other lineages.
References
Benoist, R. 1930. Descriptons d’espèces nouvelles d’Acanthacées de Madagascar. Bulletin de la Société botanique de France 76: 1031-1038.
Benoist, R. 1967. Acanthacées. In H. Humbert, Flore de Madagascar et des Comores 182, tome 1: 1-230.
Bentham, G. and J. D. Hooker. 1876. Genera Plantarum 2: 1060-1122. London: Reeve.
Daniel, T. F. 1983. Systematics of Holographis (Acanthaceae). Journal of the Arnold Arboretum 64: 129-160.
Daniel, T. F. 1985. A revision of Stenandrium (Acanthaceae) in Mexico and adjacent regions. Annals of the Missouri Botanical Garden 71: 1028-1043.
Daniel, T. F. 1991. A revision of Aphelandra (Acanthaceae) in Mexico. Proceedings of the California Academy of Sciences 47: 235-274.
Daniel, T. F. 2000. Additional chromosome numbers of American Acanthaceae. Systematic Botany 25: 15-25.
Daniel, T. F and T. I. Chuang. 1998. Chromosome numbers of cultivated Acanthaceae and systematic implications. Pp. 309-330 in Diversity and taxonomy of tropical flowering plants, eds. P. Mathew and M. Sivadasan. Calicut, India: Mentor Books.
Daniel, T. F., T. I. Chuang and M. A. Baker. 1990. Chromosome numbers of American Acanthaceae. Systematic Botany 15: 13-25.
Furness, C. A. 1990. Pollen morphology of Crossandra Salisbury and Crossandrella C. B. Clarke (Acanthaceae: Acantheae). Grana 29: 161-176.
Furness, C. A. 1994. The caveate pollen of Streptosiphon hirsutus (Acanthaceae: Acantheae) and its taxonomic significance. Kew Bulletin 49: 409-414.
Grisebach, A. 1858. Novitiae florae panamensis. Bonplandia 6: 2-12.
Grisebach, A. H. R. 1864. Flora of the British West Indian Islands. London: Reeve and Co.
Heine, H. 1966. Acanthacées. Vol. 13 in Flore de Gabon, A. Aubréville (ed.). Paris: Muséum National D’Histoire Naturelle.
Hooker, W. J. 1837. Icones Plantarum, Volume 2. London: Longman, Rees, Orme, Brown, Green and Longman.
Hooker, W. J. 1845. Curtis’s botanical magazine, Volume 71. London: Reeve Bros.
Kiel, C. A. 2006. Phylogenetic Delimitation of Isoglossinae and relationships among constituent genera. Taxon 55:3.
Leonard, E. C. 1953. The Acanthaceae of Colombia, II. Contributions from the United States National Herbarium 31: 119-322.
Mangenot, S. and G. Mangenot. 1962. Enquête sur les nombres chromosomiques dans une collection d'espèces tropicales. Revue de Cytologie et de Biologie Végetales 25: 411-447.
Manktelow, M. 1996. Phaulopsis (Acanthaceae)—a monograph. Symbolae Botanicae Upsalienses 31: 1-184.
Manktelow, M., L. A. McDade, B. Oxelman, C. A. Furness, and M.-J. Balkwill. 2001. The enigmatic tribe Whitfieldieae (Acanthaceae): delimitation and phylogenetic relationships based on molecular and morphological data. Systematic Botany 26: 104-119.
McDade, L. A. 1982. Three new species of Aphelandra (Acanthaceae) from Central America. Annals of the Missouri Botanical Garden 69: 402-411.
McDade, L. A. 1984. Systematics and reproductive biology of the Central American species of the Aphelandra pulcherrima complex (Acanthaceae). Annals of the Missouri Botanical Garden 71: 104-165.
McDade, L. A. 1992. Pollinator relationships, biogeography, and phylogenetics. BioScience 42: 21-26.
McDade, L. A. and M. L. Moody. 1999. Phylogenetic relationships among Acanthaceae: evidence from non-coding trnL-trnF chloroplast DNA sequences. American Journal of Botany 86: 70-80.
McDade, L. A. and M. D. Turner. 1997. Extrafloral nectaries in Aphelandra: anatomy, development and systematic implications. American Journal of Botany 84: 1 15.
McDade, L. A., T. F. Daniel, S. E. Masta, and K. M. Riley. 2000a. Phylogenetic relationships within the tribe Justicieae (Acanthaceae): evidence from molecular sequences, morphology, and cytology. Annals of the Missouri Botanical Garden 87: 435-458.
McDade, L. A., S. E. Masta, M. L. Moody, and E. Waters. 2000b. Phylogenetic relationships among Acanthaceae: evidence from two genomes. Systematic Botany 25: 105-120.
Moylan, E. C., J. R. Bennett, M. A. Carine, R. G. Olmstead, and R. W. Scotland. 2004. Phylogenetic relationships among Strobilanthes s.l. (Acanthaceae): evidence from ITS nrDNA, trnL-F cpDNA, and morphology. American Journal of Botany 91: 724-735.
Schwarzbach, A. E. and L. A. McDade. 2002. Phylogenetic relationships of the mangrove family Avicenniaceae based on chloroplast and nuclear ribosomal DNA sequences. Systematic Botany 27: 84-98.
Scotland, R.W. and K. Vollesen. 2000. Classification of Acanthaceae. Kew Bulletin 55: 513-589.
Scotland, R.W., J. A. Sweere, P. A. Reeves, and R. G. Olmstead. 1995. Higher-level systematics of Acanthaceae determined by chloroplast DNA sequences. American Journal of Botany 82: 266-275.
Swofford, D. L. 2000. PAUP*. Phylogenetic analysis using parsimony (*And Other Methods). Version 4. Sunderland: Sinauer Associates.
Taberlet, P., L. Gielly, G. Pautou, and J. Bouvet. 1991. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Molecular Biology 17: 1105-1109.
Tomlinson, P. B. 1986. The botany of mangroves. Cambridge: Cambridge University Press.
Vollesen, K. 1990a. Notes on Crossandra (Acanthaceae). Kew Bulletin 45:121-135.
Vollesen, K. 1990b. The genus Crossandra (Acanthaceae) in the African continent. Kew Bulletin 45: 503-534.
Vollesen, K. 1991. A revision of the African genus Sclerochiton (Acanthaceae: Acantheae). Kew Bulletin 46: 1-50.
Vollesen, K. 1992. The Old World species of Stenandrium (Acanthaceae: Acantheae). Kew Bulletin 47: 169-202.
Vollesen, K. 1994. The genus Streptosiphon (Acanthaceae: Acantheae). Kew Bulletin 9: 401-407.
Vollesen, K. 1997. Synopsis of the genus Crossandra (Acanthaceae) in Madagascar. Kew Bulletin 52: 379-415.
Vollesen, K. 1999. Cynarospermum, a new genus of Acanthaceae from India. Kew Bulletin 54: 171-177.
Vollesen, K. 2000. Blepharis (Acanthaceae): a taxonomic revision. Kew: Royal Botanic Gardens.
Wasshausen, D. C. 1975. The genus Aphelandra (Acanthaceae). Smithsonian Contributions to Botany no. 18.
Title Illustrations

| Scientific Name | Carlowrightia hintonii T.F.Daniel (Justicieae: Tetramerium Lineage) |
|---|---|
| Specimen Condition | Live Specimen |
| Copyright | © Thomas F. Daniel |
| Scientific Name | Brillantaisia nyanzarum Burkill (Ruellieae) |
|---|---|
| Location | Greenhouse, Duke University |
| Specimen Condition | Live Specimen |
| Identified By | E. A. Tripp |
| Copyright |
© 2006

|
| Scientific Name | Aphelandra impressa Lindau (Acantheae) |
|---|---|
| Location | Venezuela |
| Specimen Condition | Live Specimen |
| Identified By | L. A. McDade |
| Copyright | © 2006 Mark Skinner |
About This Page
We are grateful to Laura Schankel and Emily VanDam for a great deal of help with processing the images for the Acanthaceae ToL page. We would also like to thank all of the photographers who generously gave permission for their beautiful images to be presented here. Thanks also to the National Science Foundation (DEB 0108589 to LAM and TFD) for support of our phylogenetic work on Acanthaceae.

Rancho Santa Ana Botanic Garden, Claremont, California, USA
Carrie Kiel

Rancho Santa Ana Botanic Garden

Rancho Santa Ana Botanic Garden
Correspondence regarding this page should be directed to Lucinda A. McDade at , Carrie Kiel at , and Erin Tripp at
Page copyright © 2009 , Carrie Kiel, and
All Rights Reserved.
- First online 20 June 2005
- Content changed 15 November 2009
Citing this page:
McDade, Lucinda A., Carrie Kiel, and Erin Tripp. 2009. Acanthaceae. Version 15 November 2009. http://tolweb.org/Acanthaceae/20878/2009.11.15 in The Tree of Life Web Project, http://tolweb.org/













 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site